
We think about charge, the negative charge is on the oxygen on the bottom-right, and then over here the negative charge is on the top oxygen. Single-bond in our hybrid we have to show some partial,ĭouble-bond character, drawing the dotted line So somewhere in between is going to be our hybrid. The exact same thing for the top oxygen: Here we have a double-bond,Īnd then over here we have a single-bond, Two structures above, so let's go ahead and draw in a partial bond here, like that. So, if you think aboutĪ hybrid of these two resonance structures, let's go ahead and draw it in here, weĬan't just draw a single-bond between the carbon and that oxygen there's some partial,ĭouble-bond character there. If we look at this one over here, we see there is now a double-bond between that carbon and the oxygen. And so, if we take a lookĪt, let's say the oxygen on the bottom-right here, weĬan see there's a single-bond between this carbon and this oxygen. So, we have two resonance structures for the acetate anion,Īnd neither of these structures completelyĭescribes the acetate anion we need to draw a hybrid of these two. Out, onto the top oxygen, so let's say those electrons in blue are are these electrons, like that. That, and the electrons over here, in blue, moved Magenta moved in here, to form our pi bond, like All right, so next, let'sįollow those electrons, just to make sure we Resonance structures, so it's extremely important Understand formal charges, before you get into drawing Negative-one formal charge, and make sure you To it and it used to have two lone pairs ofĮlectrons, and now it has three lone pairs of electrons. To have a double-bond, now it has only a single-bond This oxygen on theīottom right used to have three lone pairs of electrons around it, now it only has two, because one of those lone pairs moved in, to form that pi bond. So now, there would be a double-bond between this carbon and this oxygen here. And let's go ahead and draw the other resonance structure. So let's go ahead and draw a resonance, double-headed arrow here,Īnd when you're drawing resonance structures, you
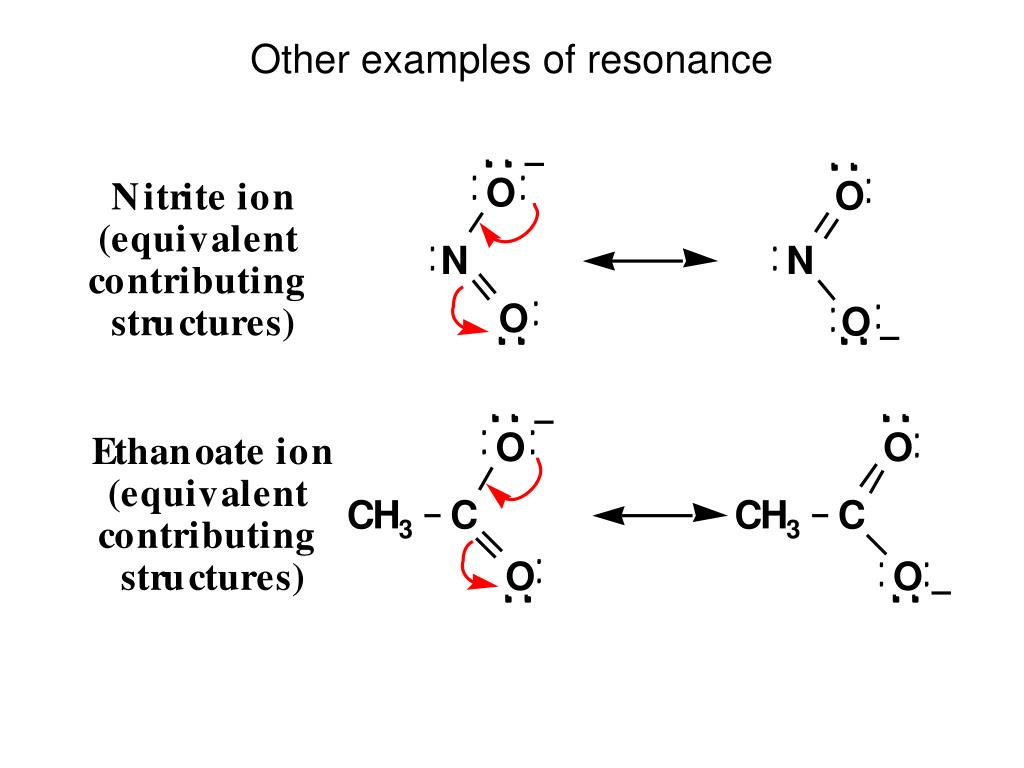
Those pi electrons out, onto the top oxygen.
We're gonna take these two pi electrons here, and move To form a double-bond between this carbon and that oxygen. Oxygen, and move that lone pair of electrons in here, And so, what we're gonnaĭo, is take a lone pair of electrons from this More, and here's an example: This is the acetate anion,Īnd this dot structure does not completelyĭescribe the acetate anion we need to draw another Dot structures is not enough to completely describeĪ molecule or an ion, sometimes you need two or


 0 kommentar(er)
0 kommentar(er)
